Each aqua blue capsule is band imprinted with the Somerset logo on the cap
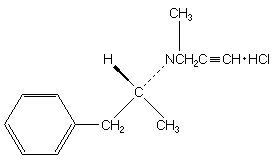
and "Eldepryl 5mg" on the body. Each capsule contains 5mg selegiline
hydrochloride. Inactive ingredients are citric acid, lactose, magnesium
stearate, and microcrystalline cellulose.
CLINICAL PHARMACOLOGY:
The mechanisms accounting for selegiline's beneficial adjunctive action in
the treatment of Parkinson's disease are not fully understood. Inhibition of
monoamine oxidase, type B, activity is generally considered to be of primary
importance; in addition, there is evidence that selegiline may act through other
mechanisms to increase dopaminergic activity.
Selegiline is best known as an irreversible inhibitor of monoamine oxidase
(MAO), an intracellular enzyme associated with the outer membrane of
mitochondria. Selegiline inhibits MAO by acting as a 'suicide' substrate for the
enzyme; that is, it is converted by MAO to an active moiety which combines
irreversibly with the active site and/or the enzyme's essential FAD cofactor.
Because selegiline has greater affinity for type B rather than for type A active
sites, it can serve as a selective inhibitor of MAO type B if it is administered
at the recommended dose.
MAOs are widely distributed throughout the body; their concentration is
especially high in liver, kidney, stomach, intestinal wall, and brain. MAOs are
currently subclassified into two types, A and B, which differ in their substrate
specificity and tissue distribution. In humans, intestinal MAO is predominantly
type A, while most of that in brain is type B.
In CNS neurons, MAO plays an important role in the catabolism of
catecholamines (dopamine, norepinephrine and epinephrine) and serotonin. MAOs
are also important in the catabolism of various exogenous amines found in a
variety of foods and drugs. MAO in the GI tract and liver (primarily type A),
for example, is thought to provide vital protection from exogenous amines (e.g.,
tyramine) that have the capacity, if absorbed intact, to cause a 'hypertensive
crisis', the so-called 'cheese reaction'. (If large amounts of certain exogenous
amines gain access to the systemic circulation - e.g., from fermented cheese,
red wine, herring, over-the-counter cough/cold medications, etc. - they are
taken up by adrenergic neurons and displace norepinephrine from storage sites
within membrane bound vesicles. Subsequent release of the displaced
norepinephrine causes the rise in systemic blood pressure, etc.)
In theory, since MAO A of the gut is not inhibited, patients treated with
selegiline at a dose of 10mg a day should be able to take medications containing
pharmacologically active amines and consume tyramine-containing foods without
risk of uncontrolled hypertension. Although rare, a few reports of hypertensive
reactions have occurred in patients receiving Eldepryl at the recommended dose,
with tyramine-containing foods. In addition, one case of hypertensive crisis has
been reported in a patient taking the recommended dose of selegiline and a
sympathomimetic medication, ephedrine. The pathophysiology of the 'cheese
reaction' is complicated and, in addition to its ability to inhibit MAO B
selectively, selegiline's relative freedom from this reaction has been
attributed to an ability to prevent tyramine and other indirect acting
sympathomimetrics from displacing norepinephrine from adrenergic neurons.
However, until the pathophysiology of the cheese reaction is more completely
understood, it seems prudent to assume that selegiline can ordinarily only be
used safely without dietary restrictions at doses where it presumably
selectively inhibits MAO B (e.g., 10mg/day).
In short, attention to the dose dependent nature of selegiline's
selectivity is critical if it is to be used without elaborate restrictions being
placed on diet and concomitant drug use although, as noted above, a few cases of
hypertensive reactions have been reported at the recommended dose. (See WARNINGS
and PRECAUTIONS.)
It is important to be aware that selegiline may have pharmacological effects
unrelated to MAO B inhibition. As noted above, there is some evidence that it
may increase dopaminergic activity by other mechanisms, including interfering
with dopamine re-uptake at the synapse. Effects resulting from selegiline
administration may also be mediated through its metabolites. Two of its three
principal metabolites, amphetamine and methamphetamine, have pharmacological
actions of their own; they interfere with neuronal uptake and enhance release of
several neurotransmitters (e.g., norepinephrine, dopamine, serotonin). However,
the extent to which these metabolites contribute to the effects of selegiline
are unknown.
Rationale for the Use of a Selective Monoamine Oxidase Type
B Inhibitor in Parkinson's Disease:
Many of the prominent symptoms of Parkinson's disease are due to a deficiency
of striatal dopamine that is the consequence of a progressive degeneration and
loss of a population of dopaminergic neurons which originate in the substantia
nigra of the midbrain and project to the basal ganglia or striatum. Early in the
course of Parkinson's Disease, the deficit in the capacity of these neurons to
synthesize dopamine can be overcome by administration of exogenous levodopa,
usually given in combination with a peripheral decarboxylase inhibitor
(carbidopa).
With the passage of time, due to the progression of the disease and/or the
effect of sustained treatment, the efficacy and quality of the therapeutic
response to levodopa diminishes. Thus, after several years of levodopa
treatment, the response, for a given dose of levodopa, is shorter, has less
predictable onset and offset (i.e., there is 'wearing off'), and is often
accompanied by side effects (e.g., dyskinesia, akinesias, on-off phenomena,
freezing, etc.).
This deteriorating response is currently interpreted as a manifestation of
the inability of the ever decreasing population of intact nigrostriatal neurons
to synthesize and release adequate amounts of dopamine.
MAO B inhibition may be useful in this setting because, by blocking the
catabolism of dopamine, it would increase the net amount of dopamine available
(i.e., it would increase the pool of dopamine). Whether or not this mechanism or
an alternative one actually accounts for the observed beneficial effects of
adjunctive selegiline is unknown.
Selegiline's benefit in Parkinson's disease has only been documented as an
adjunct to levodopa/carbidopa. Whether or not it might be effective as a sole
treatment is unknown, but past attempts to treat Parkinson's disease with
non-selective MAOI monotherapy are reported to have been unsuccessful. It is
important to note that attempts to treat Parkinsonian patients with combinations
of levodopa and currently marketed non-selective MAO inhibitors were abandoned
because of multiple side effects including hypertension, increase in involuntary
movement, and toxic delirium.
Pharmacokinetic Information (Absorption,
Distribution, Metabolism and Elimination -ADME):
The absolute bioavaliability of selegiline following oral dosing is not
known; however, selegiline undergoes extensive metabolism (presumably
attributable to presystemic clearance in gut and liver). The major plasma
metabolites are N-desmethylselegiline, L-amphetamine and L-methamphetamine. Only
N-desmethylselegiline has MAO-B inhibiting activity. The peak plasma levels of
these metabolites following a single oral dose of 10mg are from 4 to almost 20
times greater than that of the maximum plasma concentration of selegiline [1
ng/mL]. The maximum concentrations of amphetamine and methamphetamine, however,
are far below those ordinarily expected to produce clinically important effects.
Single oral dose studies do not predict multiple dose kinetics, however. At
steady state the peak plasma level of selegiline is 4 fold that obtained
following a single dose. Metabolite concentrations increase to a lesser extent,
averaging 2 fold that seen after a single dose.
The bioavailability of selegiline is increased 3 to 4 fold when it is taken
with food.
The extent of systemic exposure to selegiline at a given dose varies
considerably among individuals. Estimates of systemic clearance of selegiline
are not available. Following a single oral dose, the mean elimination half-life
of selegiline is two hours. Under steady state conditions the elimination
half-life increases to ten hours.
Because selegiline's inhibition of MAO-B is irreversible, it is impossible to
predict the extent of MAO-B inhibition from steady state plasma levels. For the
same reason, it is not possible to predict the rate of recovery of MAO-B
activity as a function of plasma levels. The recovery of MAO-B activity is a
function of de novo protein synthesis; however, information about the rate of de
novo protein synthesis is not yet available. Although platelet MAO-B activity
returns to the normal range within 5 to 7 days of selegiline discontinuation,
the linkage between platelet and brain MAO-B inhibition is not fully understood
nor is the relationship of MAO-B inhibition to the clinical effect established
(see Clinical Pharmacology).
Special Populations:
Renal Impairment:
No pharmacokinetic information
is available on selegiline or its metabolites in renally impaired subjects.
Hepatic Impairment:
No pharmacokinetic information is available on
selegiline or its metabolites in hepatically impaired subjects.
Age:
Although a general conclusion about the effects of age on the
pharmacokinetics of selegiline is not warranted because of the size of the
sample evaluated (12 subjects greater than 60 years of age, 12 subjects between
the ages of 18 to 30), systemic exposure was about twice as great in older as
compared to a younger population given a single oral dose of 10mg.
Gender:
No information is available on the effects of gender on the
pharmacokinetics of selegiline.
INDICATIONS AND USAGE:
ELDEPRYL is indicated as an adjunct in the management of Parkinsonian
patients being treated with levodopa/carbidopa who exhibit deterioration in the
quality of their response to this therapy. There is no evidence from controlled
studies that selegiline has any beneficial effect in the absence of concurrent
levodopa therapy.
Evidence supporting this claim was obtained in randomized controlled clinical
investigations that compared the effects of added selegiline or placebo in
patients receiving levodopa/carbidopa. Selegiline was significantly superior to
placebo on all three principal outcome measures employed: change from baseline
in daily levodopa/carbidopa dose, the amount of off time, and patient
self-rating of treatment success. Beneficial effects were also observed on other
measures of treatment success (e.g., measures of reduced end of dose akinesia,
decreased tremor and sialorrhea, improved speech and dressing ability and
improved overall disability as assessed by walking and comparison to previous
state).
CONTRAINDICATIONS:
ELDEPRYL is contraindicated in patients with a known hypersensitivity to this
drug.
ELDEPRYL is contraindicated for use with meperidine (DEMEROL & other
trade names). This contraindication is often extended to other opioids. (See
Drug Interactions.)
WARNINGS:
Selegiline should not be used at daily doses exceeding those recommended
(10mg/day) because of the risks associated with non-selective inhibition of MAO.
(See CLINICAL PHARMACOLOGY.)
The selectivity of selegiline for MAO B may not be absolute even at the
recommended daily dose of 10mg a day. Rare cases of hypertensive reactions
associated with ingestion of tyramine containing foods have been reported in
patients taking the recommended daily dose of selegiline. The selectivity is
further diminished with increasing daily doses. The precise dose at which
selegiline becomes a non-selective inhibitor of all MAO is unknown, but may be
in the range of 30 to 40 mg a day.
Severe CNS toxicity associated with hyperpyrexia and death have been reported
with the combination of tricyclic antidepressants and non-selective MAOIs
(NARDIL, PARNATE). A similar reaction has been reported for a patient on
amitriptyline and ELDEPRYL. Another patient receiving protriptyline and ELDEPRYL
developed tremors, agitation, and restlessness followed by unresponsiveness and
death two weeks after ELDEPRYL was added. Related adverse events including
hypertension, syncope, asystole, diaphoresis, seizures, changes in behavioral
and mental status, and muscular rigidity have also been reported in some
patients receiving ELDEPRYL and various tricyclic antidepressants.
Serious, sometimes fatal, reactions with signs and symptoms that may include
hyperthermia, rigidity, myoclonus, autonomic instability with rapid fluctuations
of the vital signs, and mental status changes that include extreme agitation
progressing to delirium and coma have been reported with patients receiving a
combination of fluoxetine hydrochloride (PROZAC) and non-selective MAOIs.
Similar signs have been reported in some patients on the combination of ELDEPRYL
(10mg a day) and selective serotonin reuptake inhibitors including fluoxetine,
sertraline and paroxetine.
Since the mechanisms of these reactions are not fully understood, it seems
prudent, in general, to avoid this combination of ELDEPRYL and tricyclic
antidepressants as well as ELDEPRYL and selective serotonin reuptake inhibitors.
At least 14 days should elapse between discontinuation of ELDEPRYL and
initiation of treatment with a tricyclic antidepressant or selective serotonin
reuptake inhibitors. Because of the long half-lives of fluoxetine and its active
metabolite, at least five weeks (perhaps longer, especially if fluoxetine has
been prescribed chronically and/or at higher doses) should elapse between
discontinuation of fluoxetine and initiation of treatment with ELDEPRYL.
PRECAUTIONS:
General:
Some patients given selegiline may experience an exacerbation of levodopa
associated side effects, presumably due to the increased amounts of dopamine
reaction with super sensitive, post-synaptic receptors. These effects may often
be mitigated by reducing the dose of levodopa/carbidopa by approximately 10 to
30%.
The decision to prescribe selegiline should take into consideration that the
MAO system of enzymes is complex and incompletely understood and there is only a
limited amount of carefully documented clinical experience with selegiline.
Consequently, the full spectrum of possible responses to selegiline may not have
been observed in pre-marketing evaluation of the drug. It is advisable,
therefore, to observe patients closely for atypical responses.
Information for Patients:
Patients should be advised of the
possible need to reduce levodopa dosage after the initiation of ELDEPRYL
therapy.
Patients (or their families if the patient is incompetent) should be advised
not to exceed the daily recommended dose of 10mg. The risk of using higher daily
doses of selegiline should be explained, and a brief description of the cheese
reaction provided. Rare hypertensive reactions with selegiline at recommended
doses associated with dietary influences have been reported.
Consequently, it may be useful to inform patients (or their families) about
the signs and symptoms associated with MAOI induced hypertensive reactions. In
particular, patients should be urged to report, immediately, any severe headache
or other atypical or unusual symptoms not previously experienced.
Laboratory Tests:
No specific laboratory tests are deemed essential
for the management of patients on ELDEPRYL. Periodic routine evaluation of all
patients, however, is appropriate.
Drug Interactions:
The occurrence of stupor, muscular rigidity,
severe agitation, and elevated temperature has been reported in some patients
receiving the combination of selegiline and meperidine. Symptoms usually resolve
over days when the combination is discontinued. This is typical of the
interaction of meperidine and MAOIs. Other serious reactions (including severe
agitation, hallucinations, and death) have been reported in patients receiving
this combination (see CONTRAINDICATIONS). Severe toxicity has also been
reported in patients receiving the combination of tricyclic antidepressants and
ELDEPRYL and selective serotonin reuptake inhibitors and ELDEPRYL. (See
WARNINGS for details.) One case of hypertensive crisis has been reported
in a patient taking the recommended doses of selegiline and a sympathomimetic
medication (ephedrine).
Carcinogenesis, Mutagenesis, and Impairment of
Fertility:
Assessment of the carcinogenic potential of selegiline in mice
and rats is ongoing.
Selegiline did not induce mutations or chromosomal damage when tested in the
bacterial mutation assay in Salmonella typhimurium and in an in vivo
chromosomal aberration assay. While these studies provide some reassurance that
selegiline is not mutagenic or clastogenic, they are not definitive because of
methodological limitations. No definitive in vitro chromosomal aberration
or in vitro mammalian gene mutation assays have been performed.
The effect of selegiline on fertility has not been adequately assessed.
Pregnancy:
Pregnancy Category C: No teratogenic effects were
observed in a study of embryo-fetal development in Sprague-Dawley rats at oral
doses of 4, 12, and 36 mg/kg or 4, 12 and 35 times the human therapeutic dose on
a mg/m2 basis. No teratogenic effects were observed in a study of
embryo-fetal development in New Zealand White rabbits at oral doses of 5, 25,
and 50 mg/kg or 10, 48, and 95 times the human therapeutic dose on a
mg/m2 basis; however, in this study, the number of litters produced
at the two higher doses was less than recommended for assessing teratogenic
potential. In the rat study, there was a decrease in fetal body weight at the
highest dose tested. In the rabbit study, increases in total resorptions and %
post-implantation loss, and a decrease in the number of live fetuses per dam
occurred at the highest dose tested. In a peri-and postnatal development study
in Sprague-Dawley rats (oral doses of 4, 16, and 64 mg/kg or 4, 15, and 62 times
the human therapeutic dose on a mg/m2 basis), an increase in the
number of stillbirths and decreases in the number of pups per dam, pup survival,
and pup body weight (at birth and throughout the lactation period) were observed
at the two highest doses. At the highest dose tested, no pups born alive
survived to Day 4 postpartum. Postnatal development at the highest dose tested
in dams could not be evaluated because of the lack of surviving pups. The
reproductive performance of the untreated offspring was not assessed.
There are no adequate and well-controlled studies in pregnant women.
Selegiline should be used during pregnancy only if the potential benefit
justifies the potential risk to the fetus.
Nursing Mothers:
It is not known whether selegiline hydrochloride
is excreted in human milk. Because many drugs are excreted in human milk,
consideration should be given to discontinuing the use of all but absolutely
essential drug treatments in nursing women.
Pediatric Use:
The effects of selegiline hydrochloride in children
have not been evaluated.
ADVERSE REACTIONS:
Introduction:
The number of patients who received selegiline in
prospectively monitored pre-marketing studies is limited. While other sources of
information about the use of selegiline are available (e.g., literature reports,
foreign post-marketing reports, etc.) they do not provide the kind of
information necessary to estimate the incidence of adverse events. Thus, overall
incidence figures for adverse reactions associated with the use of selegiline
cannot be provided. Many of the adverse reactions seen have also been reported
as symptoms of dopamine excess.
Moreover, the importance and severity of various reactions reported often
cannot be ascertained. One index of relative importance, however, is whether or
not a reaction caused treatment discontinuation. In prospective pre-marketing
studies, the following events led, in decreasing order of frequency, to
discontinuation of treatment with selegiline: nausea, hallucinations, confusion,
depression, loss of balance, insomnia, orthostatic hypotension, increased
akinetic involuntary movements, agitation, arrhythmia, bradykinesia, chorea,
delusions, hypertension, new or increased angina pectoris, and syncope. Events
reported only once as a cause of discontinuation are ankle edema, anxiety,
burning lips/mouth, constipation, drowsiness/lethargy, dystonia, excess
perspiration, increased freezing, gastrointestinal bleeding, hair loss,
increased tremor, nervousness, weakness, and weight loss.
Experience with ELDEPRYL obtained in parallel, placebo controlled, randomized
studies provides only a limited basis for estimates of adverse reaction rates.
The following reactions that occurred with greater frequency among the 49
patients assigned to selegiline as compared to the 50 patients assigned to
placebo in the only parallel, placebo controlled trial performed in patients
with Parkinson's disease are shown in the following Table. None of these adverse
reactions led to a discontinuation of treatment.
|
| INCIDENCE OF TREATMENT-EMERGENT ADVERSE
EXPERIENCES IN THE PLACEBO-CONTROLLED CLINICAL TRIAL |
|
| Adverse Event |
Number of Patients Reporting
Events |
|
selegiline hydrochloride |
placebo |
|
N=49 |
N=50 |
| Nausea |
10 |
3 |
| Dizziness/Lightheaded/Fainting |
7 |
1 |
| Abdominal Pain |
4 |
2 |
| Confusion |
3 |
0 |
| Hallucinations |
3 |
1 |
| Dry mouth |
3 |
1 |
| Vivid Dreams |
2 |
0 |
| Dyskinesias |
2 |
5 |
| Headache |
2 |
1 |
|
| The following events were reported once in either or both
groups |
| Ache, generalized |
1 |
0 |
| Anxiety/Tension |
1 |
1 |
| Anemia |
0 |
1 |
| Diarrhea |
1 |
0 |
| Hair Loss |
0 |
1 |
| Insomnia |
1 |
1 |
| Lethargy |
1 |
0 |
| Leg pain |
1 |
0 |
| Low back pain |
1 |
0 |
| Malaise |
0 |
1 |
| Palpitations |
1 |
0 |
| Urinary Retention |
1 |
0 |
| Weight Loss |
1 |
0 |
|
In all prospectively monitored clinical investigations, enrolling
approximately 920 patients, the following adverse events, classified by body
system, were reported.
Central Nervous
System:
Motor/Coordination/Extrapyramidal:
increased
tremor, chorea, loss of balance, restlessness, blepharospasm, increased
bradykinesia, facial grimace, falling down, heavy leg, muscle
twitch*, myoclonic jerks*, stiff neck, tardive dyskinesia,
dystonic symptoms, dyskinesia, involuntary movements, freezing, festination,
increased apraxia, muscle cramps.
Mental Status/Behavioral/Psychiatric:
hallucinations,
dizziness, confusion, anxiety, depression, drowsiness, behavior/mood change,
dreams/nightmares, tiredness, delusions, disorientation, lightheadedness,
impaired memory*, increased energy*, transient
high*, hollow feeling, lethargy/malaise, apathy, overstimulation,
vertigo, personality change, sleep disturbance, restlessness, weakness,
transient irritability.
Pain/Altered Sensation:
headache, back pain, leg pain,
tinnitus, migraine, supraorbital pain, throat burning, generalized ache, chills,
numbness of toes/fingers, taste disturbance.
Autonomic Nervous System:
dry mouth, blurred vision, sexual
dysfunction.
Cardiovascular:
orthostatic hypotension, hypertension, arrhythmia,
palpitations, new or increased angina pectoris, hypotension, tachycardia,
peripheral edema, sinus bradycardia, syncope.
Gastrointestinal:
nausea/vomiting, constipation, weight loss,
anorexia, poor appetite, dysphagia, diarrhea, heartburn, rectal bleeding,
bruxism*, gastrointestinal bleeding (exacerbation of preexisting
ulcer disease).
Genitourinary/Gynecologic/Endocrine:
slow urination, transient
anorgasmia*, nocturia, prostatic hypertrophy, urinary hesitancy,
urinary retention, decreased penile sensation*, urinary frequency.
Skin and Appendages:
increased sweating, diaphoresis, facial hair,
hair loss, hematoma, rash, photosensitivity.
Miscellaneous:
asthma, diplopia, shortness of breath, speech
affected.
Postmarketing Reports:
The following experiences were described in
spontaneous post-marketing reports. These reports do not provide sufficient
information to establish a clear causal relationship with the use of ELDEPRYL.
CNS:
Seizure in dialyzed chronic renal failure patient on
concomitant medications.
* indicates events reported only at doses greater than 10mg/day.
OVERDOSAGE:
Selegiline:
No specific information is available about clinically
significant overdoses with ELDEPRYL. However, experience gained during
selegiline's development reveals that some individuals exposed to doses of 600
mg of d,l-selegiline suffered severe hypotension and psychomotor agitation.
Since the selective inhibition of MAO B by selegiline hydrochloride is
achieved only at doses in the range recommended for the treatment of Parkinson's
disease (e.g., 10mg/day), overdoses are likely to cause significant inhibition
of both MAO A and MAO B. Consequently, the signs and symptoms of overdose may
resemble those observed with marketed non-selective MAO inhibitors [e.g.,
tranylcypromine (PARNATE), isocarboxazide (MARPLAN), and phenelzine (NARDIL)].
Overdose with Non-Selective MAO Inhibition:
NOTE:
This section
is provided for reference; it does not describe events that have actually been
observed with selegiline in overdose.
Characteristically, signs and symptoms of non-selective MAOI overdose may not
appear immediately. Delays of up to 12 hours between ingestion of drug and the
appearance of signs may occur. Importantly, the peak intensity of the syndrome
may not be reached for upwards of a day following the overdose. Death has been
reported following overdosage. Therefore, immediate hospitalization, with
continuous patient observation and monitoring for a period of at least two days
following the ingestion of such drugs in overdose, is strongly recommended.
The clinical picture of MAOI overdose varies considerably; its severity may
be a function of the amount of drug consumed. The central nervous and
cardiovascular systems are prominently involved.
Signs and symptoms of overdosage may include, alone or in combination, any of
the following: drowsiness, dizziness, faintness, irritability, hyperactivity,
agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions,
and coma; rapid and irregular pulse, hypertension, hypotension and vascular
collapse; precordial pain, respiratory depression and failure, hyperpyrexia,
diaphoresis, and cool, clammy skin.
Treatment Suggestions For Overdose:
NOTE:
Because there is no
recorded experience with selegiline overdose, the following suggestions are
offered based upon the assumption that selegiline overdose may be modeled by
non-selective MAOI poisoning. In any case, up-to-date information about the
treatment of overdose can often be obtained from a certified Regional Poison
Control Center. Telephone numbers of certified Poison Control Centers are listed
in the Physicians Desk Reference (PDR).
Treatment of overdose with non-selective MAOIs is symptomatic and supportive.
Induction of emesis or gastric lavage with instillation of charcoal slurry may
be helpful in early poisoning, provided the airway has been protected against
aspiration. Signs and symptoms of central nervous system stimulation, including
convulsions, should be treated with diazepam, given slowly intravenously.
Phenothiazine derivatives and central nervous system stimulants should be
avoided. Hypotension and vascular collapse should be treated with intravenous
fluids and, if necessary, blood pressure titration with an intravenous infusion
of a dilute pressor agent. It should be noted that adrenergic agents may produce
a markedly increased pressor response.
Respiration should be supported by appropriate measures, including management
of the airway, use of supplemental oxygen, and mechanical ventilatory
assistance, as required.
Body temperature should be monitored closely. Intensive management of
hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is
essential.
DOSAGE AND ADMINISTRATION:
ELDEPRYL is intended for administration to Parkinsonian patients receiving
levodopa/carbidopa therapy who demonstrate a deteriorating response to this
treatment. The recommended regimen for the administration of ELDEPRYL is 10mg
per day administered as divided doses of 5mg each taken at breakfast and lunch.
There is no evidence that additional benefit will be obtained from the
administration of higher doses. Moreover, higher doses should ordinarily be
avoided because of the increased risk of side effects.
After two to three days of selegiline treatment, an attempt may be made to
reduce the dose of levodopa/carbidopa. A reduction of 10 to 30% was achieved
with the typical participant in the domestic placebo controlled trials who was
assigned to selegiline treatment. Further reductions of levodopa/carbidopa may
be possible during continued selegiline therapy.
HOW SUPPLIED:
ELDEPRYL capsules are available containing 5mg of selegiline hydrochloride.
Each aqua blue capsule is band imprinted with the Somerset logo on the cap and
"Eldepryl 5mg" on the body.
They are available as:
NDC 39506-022-60 bottles of 60 capsules.
NDC
39506-022-30 bottles of 300 capsules.
Store at controlled room temperature, 59° to 86°F (15° to 30°C).
CAUTION: Federal (USA) law prohibits dispensing without prescription.
Somerset Pharmaceuticals, Inc.
Tampa, FL 33607
Literature
issued February 1997
ELD:R13